What’s inside:
The FDA regulates the automatic tracking of implants, tissue and medical devices. This article looks at:
- The background to UDI device tracking
- The reason for implant tracking
- The connection between UDI medical device tracking and patient safety
- The challenges faced documenting non-sterile orthopedic implants
- The GS1 US White Paper on Examining UDI Capture & Orthopedic Implants
- The use of technology in tracking non sterile surgical medical supplies
UDI management of high-value inventory
The FDA’s UDI regulations are designed to support healthcare medical device and implant tracking, from the point they are delivered, right through to patient consumption.
UDI provides a standardized method for healthcare information systems to identify and record medical devices in electronic health records (EHR), claims, and devices registries.
What is UDI device tracking?
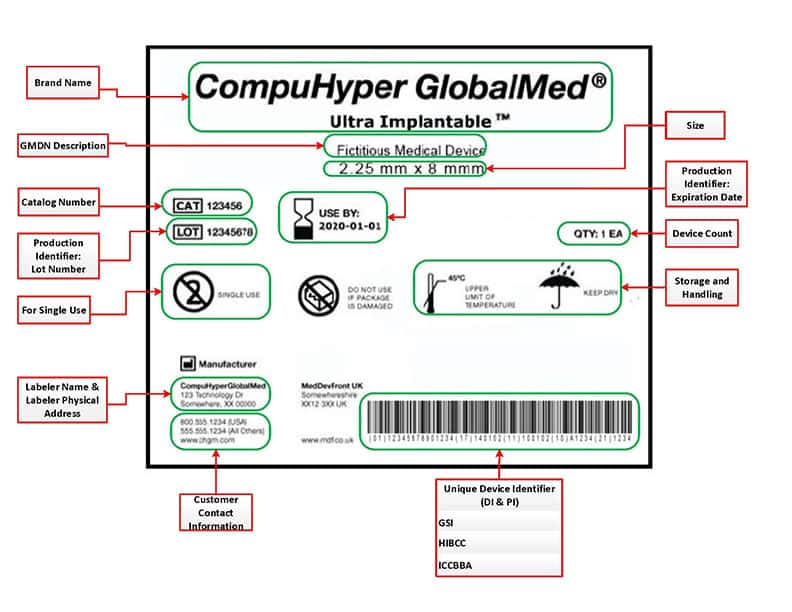
UDI stands for Unique Device Identifier and is made up of a standardized and unique numeric or alphanumeric identification code, which is assigned to medical device products by the manufacturer.
UDI included two parts:
1. A DI or ‘Device Identifier’ which is a mandatory, fixed portion of a UDI code that represents the manufacturer and the specific version/model of the device. For organizations using the GS1 Standards, the DI is represented by the Global Trade Item Number or ‘GTIN’.
2. A PI or ‘Production Identifier which is a conditional, variable portion of a UDI that identifies one or more of the following
-
- lot or batch number
- serial number
- expiration date
- date of manufacture
- the distinct identification code (for an HCT/P regulated as a devices)
The UDI Rule states that a Device Identifier must always be recorded, but a Production Identifier is only required if it appears on a device label. However, at the point that the regulations were issued, most implants included at least one piece of production information on the label, so the full UDI for these items was required for documentation.
Why are implants tracked?
Implant tracking needs to take place from the moment the item reaches the healthcare provider until it is used in surgery.
That means that there needs to be an efficient system in place:
- to automatically track implants while they are in storage
- at the point of care when the implants will be used
The most vital point in the process is to capture utilization.
UDI device tracking and patient safety
Implants are tracked because after consumption there needs to be a way to collate data on any complications, roll out design updates and, most importantly, deal with any future product recalls.
When a product recall is issued there needs to be a swift ‘track and trace’ operation so that all patients affected can be quickly identified and contacted. The 2019 Allergan breast implant recall highlighted the importance of UDI tracking, when 52,000 patients who had received the implant were unable to be tracked due to system inefficiencies. The case put the spotlight on the need for UDI batch tracking to improve patient safety and save lives.
UDI device tracking and organizational efficiency
Another benefit to tracking UDI medical devices is that the data collected can be leveraged so that the healthcare provider can better understand inventory management and utilization, so that they can learn from their data to improve operational and clinical planning.
When UDI is digitally captured at the point of care it helps the organization to add efficiency to inventory management and wastage, with the potential to make significant cost savings and improve the quality of patient care.
Robust implant tracking enables healthcare providers to quickly respond to product recalls, preventing both patient risk and pricy litigation.
How is UDI medical device tracking achieved?
Since the launch of the UDI regulations for healthcare, hospitals and surgery centers have been integrating the tracking of their medical supplies into hospital workflows, so that stock items be digitally tracked and quickly located. There are a range of manual and automated systems in place which aim to achieve full implant tracking. Automated UDI tracking systems are seen as the best option for achieving the real time tracking of implants, tissue and medical devices – but even these systems have their challenges.
When it comes to non-sterile items, the system can break down, as packaging often becomes separated from items before any digital connection has been made between the patient and the product.
Once this happens there is a high risk of the item being consumed without any association being made between the product and the patient.
Non-sterile orthopedic implants (e.g., screws, plates, etc.) can present a real challenge for hospitals as these items cannot be tracked using the hospital’s standard workflows and run the high risk of being untracked. If documentation is not achieved then, should the product later be recalled, there is no way to identify that a patient has consumed the item, and therefore no way to contact them and take the required action, putting their safety at risk.
GS1 White Paper on orthopedic challenges with UDI capture
In 2016 the GS1 US Orthopedic Implant Workgroup was established to analyze the challenges that healthcare providers were experiencing when attempting to log UDI orthopedic devices at the point of use.
The Workgroup consisted of device manufacturers, healthcare providers, and heath-tech companies and were tasked with evaluating UDI capture options, while assessing both the benefits and challenges from all perspectives ie. manufacturers and providers.
The Workgroup analyzed data and device process flows plus set up site visits to hospitals which included observing live orthopedic surgeries so that they could gain a better understanding of the complexity of UDI capture in the operating room (OR) environment. Once the research stage was complete the Working Group looked into the options available, assessing them for feasibility and looking at both the challenges and benefits, again viewed from both provider and vendor perspectives.
This work resulted in the GS1 US White Paper ‘Examining UDI Capture & Orthopedic Implants which aims to provide insight and help the sector move forward with solutions to the challenges they identified.

Tracking non-sterile orthopedic implants
The GS1 working group found that the tracking of non-sterile implants in orthopedic surgeries presents unique challenges in terms of UDI processes. Let’s look at why.
The standard process of non-sterile orthopedic implants
Hospital sterilized orthopedic implants are delivered by vendors in an unsterile state and are then sterilized by the hospital prior to usage.
These small devices come in many types, varieties and sizes, and are commonly stored in sets or trays which hold hundreds of devices, ready for assembly in the procedure.
The entire tray will be sterilized at the hospital prior to a procedure, with the surgeon then selecting just those items required during the surgery. The remaining items are then disinfected and returned to storage, where the missing items will be replenished by reps on-site, and the whole cycle will begin again with the tray being sent to the hospital’s sterilizing unit when the kit is needed for another surgery.
Challenges of UDI tracking of non-sterile items in the OR setting
There are several challenges that make UDI tracking of these small surgical medical supplies difficult:
- The UDI code can be lost when the packaging is removed as it is placed on the orthopedic tray ready for sterilization
- UDI codes cannot be marked directly onto these items due to issues with their size, shape and material components, so the packaging is the last link to a product’s UDI.
- The point of use has specific data capture challenges connected to patient safety.
The GS1 Working Group looked at the challenges that non-sterile UDI devices pose in the surgical setting.
Recommendations of the GS1 US Working Group
The GS1 US Working Group on non-sterile orthopedic implants encouraged the FDA and industry to work together toward a solution to improve the UDI capture of orthopedic kits.
In March 2016, the FDA confirmed that, “capture of the DI alone meets the objectives of the UDI rule for those non-sterile implantable devices where it would not be technologically feasible to capture the full UDI at point of implantation.”
In addition, the FDA also confirmed that, “using cross-reference tools (like Inventory Control Sheets) to capture the DI were acceptable methodologies until technologies to enable capture of the full UDI become available.”
The FDA noted that the “industry will continue to develop technologies for making the full UDI available at the point of use that do not rely on indirect methods such as cross-reference tools, and that they look forward to receiving updates on industry’s progress.”
Read on to see how AI technology is achieving full UDI documentation at the point of use.
How technology is being used to record orthopedic sets
The surgical workflow should allow for devices that are used during procedures to have their UDIs recorded, either manually or using technology. Because manual data entry is known to be error-prone, automation is seen as the more efficient method of UDI capture.
In some hospitals OR nurses are able to set up an online Inventory Control Sheet before surgery which includes the required UDI data for each device listed. During surgery the nurse will note the quantity of each item used and this can then be manually entered, or barcode scanned on to the patient record.
Although this is certainly a step up, it is not without its challenges:
- Setting up the online inventory control sheet is time consuming
- Manual data entry is a slow, error-prone method of capturing UDI devices in surgery
- Barcode scans often fail – the GS1 work group found that the root cause of this issue is usually the organization’s own data management system, as opposed to a barcode issue for example it could be that less commonly used devices simply aren’t listed in hospital systems.
- When scanning fails nurses’ resort to keying the data in manually.
It’s clear that a different approach is necessary if the accurate recording of Unique Device Identifiers is to be achieved. One that takes account of the complex nature of work in the OR setting and where UDI capture does not detract from patient care.
How image recognition technology can support the UDI capture of non-sterile orthopedic items
New technology is now being used to approach the issue in a different way.
‘Snap and Go’ uses image recognition technology as the method of implant data capture.
For the majority of items, it simply takes a quick ‘snap’ of the packaging and then uses AI and ML technology to identify and document each item, straight into hospital systems.
With non-sterile items the absence of a package prompted a different approach.
‘Snap & Go’ is now the only tool on the market that uses computer vision to ‘read’ manually completed implant sheets.
The Circulating Nurse simply needs to manually note down all items used on the implant sheet during surgery and then take a ‘snap’ of the completed sheet.
‘Snap & Go’ is powered by AI technology and is also backed up by a global SKU database. It is able to identify and document every item in the quantities used, and record this data directly into the EHR, ERP and MMIS – ensuring that surgical charting is complete and correct, medical billing is accurate and that the implant usage is noted by the inventory management software.
This patent-protected software is the first of its kind and makes the digital recording of manually completed Implant Sheets an easy to implement reality.

Capturing Unique Device Identifier Data on Non-Sterile Orthopedic Implants
The GS1 US Working Group recognized the complexities of data capture in the OR setting and the particular challenges associated with managing non-sterile orthopedic implants efficiently.
It’s clear that patient safety is the primary focus of the nurse in OR and procedure rooms. Any data capture tool has to be ‘admin-light’ so as not to distract her from patient care.
The GS1 Working Group recommended the continued development of data capture solutions for non-sterile orthopedic implants in the OR setting.
‘Snap & Go’ is the new technology designed especially for the orthopedic setting – finally providing a quick and simple way for nurses to digitally document FULL UDI data of ALL implants used in surgery, freeing them up to focus on patient care.
Contact us to find out how ‘Snap & Go’ can help capture UDI data on your non-sterile orthopedic devices.







