In the summer of 2019, the U.S. Food and Drug Administration (FDA) requested that manufacturer Allergan recall specific models of its textured breast implants from the U.S. market due to the risk of BIA-ALCL. The FDA at the time was aware that 573 cases and 12 deaths occurred when the defective implants were known to be implanted in patients.
The scandal began following that request by the FDA to notify the public that it was moving forward with a worldwide recall of the BIOCELL textured breast implant products as well as tissue expanders but surprisingly only 10 months afterward, the company publicly acknowledged that it does not have device tracking information for some 52,000 breast implants.
Why Were the Textured Breast Implants Recalled?
Through ongoing research of BIA-ALCL and examination of confirmed medical device reports, there was evidence that the texturizing process used for some breast implants increased the risk of developing Anaplastic large cell lymphoma. The FDA requested a voluntary recall of the Biocell surface devices in the United States. The recall was a Class I recall, which is the most serious type of recall. Recalls may fall under these categories:
Class I recall: There is a reasonable probability that the use of or exposure to a product will cause serious adverse health consequences or death.
Class II recall: The use of or exposure to a product may cause temporary or medically reversible adverse health consequences.
Class III recall: The use of or exposure to a violative product is not likely to cause adverse health consequences.
What is Anaplastic Large Cell Lymphoma (ALCL)?
The defective implants breast implants have an outer shell of silicone elastomer, which is a type of silicone with elastic properties, very similar to rubber. These shells can be filled with sterile saline, which is a saltwater solution, or with silicone gel.
Breast implant-associated anaplastic large cell lymphoma (BIA-ALCL) is an uncommon and highly treatable type of lymphoma that can develop around breast implants. Most of the cases of BIA-ALCL occur in patients who have breast implants with textured surfaces.
A diagnosis of ALCL requires taking a biopsy and looking at the cells under a microscope. Additional tests such as blood tests, a CT scan, a PET scan, an MRI scan, and bone marrow biopsy, may be required as well.
Common symptoms associated with ALCL
- Breast pain
- Swelling around the breast
- Seroma
- Rash on breast skin
- Mass or lump around the implant
- Redness
- Changes in breast size and shape
How Do Breast Implants Get Recalled?
The FDA established a unique device identification system, also known as UDI, to adequately identify medical devices from manufacturing through distribution to patient use, requiring the device labels to bear a globally unique device identifier that is digitally stored. When the system is fully implemented, the label of most devices will include a unique device identifier (UDI) in human and machine-readable form, which will ultimately improve patient safety, modernize device postmarket surveillance, and facilitate medical device innovation.
The UDI is a unique numeric or alphanumeric code related to a medical device and comprises the following components:
- A device identifier (UDI-DI)
- A production identifier (UDI-PI)
The capture of UDI at the point of clinical care is the linchpin for UDI use for clinical purposes and recall cases. This will be done utilizing automatic identification and data capture (AIDC). AIDC can be any technology that conveys the UDI or the device identifier in a form that can be entered into an electronic patient record or another computer system via an automated process.
The data will then be populated in a Global Unique Device Identification Database (GUDID) that will be a true data source of all medical devices.

What is the purpose of UDI?
The main purpose of UDI is to make medical devices easier to identify to help reduce medical errors, fight against falsified devices and most importantly, be able to locate patients affected by a recall and provide them with assistance and maintain accurate tracking.
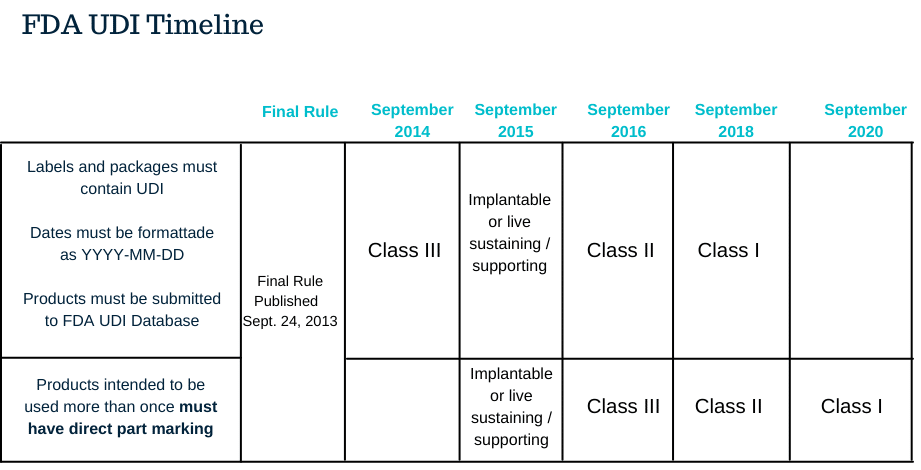
The FDA established key compliance dates for all other provisions of the final rule, which divide all the devices into three groups according to the levels of risk and urgency.
Since 1980, breast implants are classified into Class III, higher-risk products. As of 2016, Class III devices must bear a UDI as a permanent marking on the device itself.
Why it was Difficult to Track all Recalled Breast Implants?
In 2020, The Department of Plastic and Reconstructive Surgery, University of Montreal Hospital, Canada, released a study criticizing the recall execution due to difficulties in identifying patients affected by the recall.
First, the study highlights the utmost importance of building a national implants registry in the event of another recall. This recommendation is motivated by the difficulties encountered in identifying patients affected by the recall. On the one hand, patients’ surgeons and places of residence change over the years, with many of the women moving across the country and becoming harder to reach and notify. On the other hand, a significant lack of implant traceability in systems belonging respectively to the company and the hospital considerably hindered data collection.
During the recall process, it was revealed that the brand name has changed over time: McGhan and Inamed. Therefore, the study believes that implants should be identified based on their characteristics rather than their brand name.
Needless to say, in the global and digital world, there is no room for the manual and local registry. Technologies that are not able to support it must be replaced.
Snap & Go – UDI Capture by Image Capture For Future Recalls
IDENTI Medical set out to address this problem with the introduction of Snap & Go: an advanced image processing and machine learning system that records visual proof for safety protocols.
Unlike barcode scanning, Snap & Go captures all pertinent information just by identifying a photo image of the item. In a few seconds, Snap&Go quickly checks the expiration date, lot/batch and serial number makes sure everything is safe, and then automatically records the product in the patient’s chart. A centralized database batch/lot history is kept, making it easier to locate defective recalled products in a simple computer query.
The case of Allergan recalled breast implants demonstrates the direct impact of accurate product information capture on patient safety. If only full digital documentation of batch numbers had been made in the transplant patients’ files, the names of the women could have been extracted using an electronic tracking system.
Snap&Go’s cloud software easily integrates into the hospital’s systems, with a simple one-time implementation, making sure all batch history is retained for future recalls. The system is quick and easy for the OR staff to use and offers full visibility, automatically sending relevant data to EHR and ERP systems with minimal intervention from the OR team.
There is currently no other solution that can assist medical staff at the point-of-use in dealing with such cases, and again they are left exposed to legal action. It’s time to stop scanning and start snapping.






