What’s inside:
This article discusses the reasons why UDI codes change, and the impact this can have on healthcare organizations.
- We break down the two numerical sections of the UDI code and learn the circumstances that trigger alterations.
- We discuss the difficulty that UDI changes pose for healthcare organizations who, under FDA UDI regulations, are required to track all medical devices and implants from delivery right through to the point of use.
- We look at how UDI changes affect barcode readability making implant tracking and recording a difficult task.
The FDA UDI regulations
The FDA set up the Unique Device Identification (UDI) system to aid the identification of products ‘from manufacturing, through distribution, to patient use’.
The UDI system applies to all medical devices and implants, except custom-made and performance study/investigational devices. The system involves the issuing of UDI numbers, which are centrally recorded and then included on the product label.
UDI tracking in healthcare is important. Coded labels help organizations to identify and track healthcare products, such as medical devices and implants, as they move through the supply chain and on to the end user.
This multi-agency data synergy enhances patient safety and improves supply chain efficiency.
UDI Implant Labelling
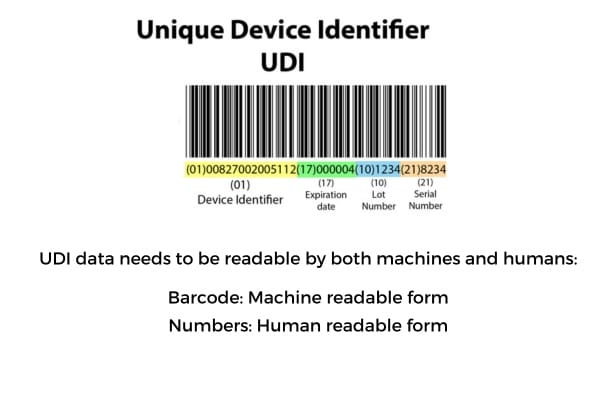
The UDI implant system facilitates the unambiguous identification of medical devices – the UDI code is made up of the UDI-DI and UDI-PI:
- Device Identifier (DI)
- Product Identifier (PI)
The device manufacturer has the responsibility for complying with UDI requirements, including the assignment of unique UDIs – both the DI and PI.
The DI section of the UDI code is mandatory, and the number needs to be issued by an FDA-approved entity. This portion of the barcode is generally thought of as static data.
The PI code includes the DI number as its prefix, followed by additional information on the product, which is subject to change, for example the batch number and expiration date.
GUDID Database
GUDID stands for Global Unique Device Identification Database. This is a database that is administered by the FDA and acts as a reference catalog for every UDI – covering all medical devices that have a UDI allocated to them.
GUDID just logs the UDI-DI part of the coding. No UDI-PI is listed in GUDID.
GS1 UDI issuing agency
DI numbers are issued by external agents. There are several entities that are approved by the FDA to assign UDIs, and a popular issuing agency in the US is GS1.
GS1 has healthcare-specific standards in place. The GS1 Standards in Healthcare support vendors, distributors, and healthcare organizations to use a consistent, globally recognized product labelling system for medical devices, implants and consumables.
GS1 issues the Device Identifier, in the form of Global Trade Item Numbers (GTINs). These numbers are produced in an internationally recognized barcode format, made up of a unique 14-digit number.
Changes to UDI Device Identifier
The format of the Device Identifier varies depending on the issuing agent.
We will be looking at the GS1 GTIN number is an example of a Device Identifier.
The GTIN DI number has four sections which can be broken down as:
- The GS1 Prefix – for the country of origin
- The Company Prefix – a unique reference number
- The Item Reference – the product type
- The Check Digit – ensures correct code composition.
Although often referred to as fixed data, there are circumstances when the DI is subject to change.
While some products may keep the same barcode for years, others may go through changes which lead them to be classed as ‘replacement products’, which require a new GTIN.
The GTIN Management Rules include guidance on when an existing product’s changing attributes will result in the need to create a new GTIN.
Let’s take a look at some examples of product changes that lead to a new GTIN being issued on an existing item.
What are the most common reasons that Device Identifiers (DI) change?
A new UDI-DI needs to be assigned to a product when there is any change that could result in the misidentification of the device or affect its traceability.
There are many reasons that the UDI product identifier, or GTIN, requires a replacement GTIN.
Sometimes the reason may relate to a change in the UDI implant itself, but it could also be an alteration in the packaging.
Below are some examples of changes that require a change of DI:
- Change in specifications, performance, size or composition of the device, to an extent that is greater than the specified limits. This can include the packaging itself.
- Change in the quantity of devices contained within a package, or the addition of a new device package
- Change from a non-sterile package to a sterile package, or vice versa
- Relabeling of the original device
- Change relating to a different language on the label, to suit different global markets
- Change in certification mark eg. CE Mark
- Change of dimensions in outer packaging
We can see that although the UDI Device Identifier is often thought of as fixed, in reality there are many circumstances when a new number needs to be issued for that product.
So, the DI is subject to change – but does this really matter?
The answer is a definite yes!
Dealing with changes to UDI
Organizations across the supply chain need to be kept aware of changes to UDIs so that they can update their systems.
For an item to be read and identified, the mandatory DI section of the UDI needs to be listed on the organization’s ‘source of truth’. In healthcare organizations this is often referred to as the ‘Item Master’. This is a long list of products, documenting key information, including the Device Identifier.
The Item Master is used by the hospital system as source data for identifying an item when it is scanned or manually entered onto the system.
With hundreds of thousands of items on many Item Masters, this document needs to be carefully and regularly maintained to remain relevant.
If the DI number changes and the Item Master does not keep pace with the change, then this will cause UDI readability issues.
However, hospital Item List maintenance is far from simple, and it often feels like ‘chasing your tail’.
With so new suppliers, new products plus changes to the UDI on regular stock items, keeping the hospital system up to date is an almost impossible task.
What are the repercussions of the UDI code alterations?
In order to understand the impact that UDI changes have on healthcare organizations, let’s take a look at an example of UDI tracking in practice.
We are going to consider the effect that a new UDI code might have on the recording of utilization at the point of care.
This is a crucial task that provides a digital link between products and patients.
If a product used during surgery has undergone changes which resulted in a new Device Identifier being assigned to it, and if this new code has not been updated on the Item Master – then when the product is scanned at the point of use, it won’t be recognized by the system.
When this happens there are two likely outcomes:
- There is a time-consuming, complex workaround involving several staff, to get the item correctly listed on the hospital’s system. Once loaded onto Item Master, the product can then be successfully scanned, identified and recorded.
- The system falls down at any given point and the item ends up not being recorded at all.
If UDI changes sometimes result in the non-documentation of items at the point of care, what are the ramifications?
The impact of non-documented items
Recording utilization at the point of care is a crucial task.
When medical devices and implants are not recorded there are implications for patient safety, inventory management and billing.
Patient Safety
If a future medical device product recall occurs and there is no digital link made between the item and the patient, then the patient will not be identified using standard electronic track and trace reporting.
The item may have been recorded manually in ‘the big book’, with the label being stuck in as a record. But without electronic indexing, manually tracking down patients affected by a medical product recall is time-consuming and inefficient.
Not documenting medical devices is a UDI tracking fail, which is a non-compliant act that goes against the FDA UDI regulations, which healthcare organizations and nurses are bound by.
In 2023 all healthcare organizations need to have a process in place to systematically collect and digitally record ALL items used in surgery.
Non documentation is a serious patient risk.
Inventory Management
Unrecorded items remain invisible to the materials management team.
Non documentation means that items used in surgery are invisible to the materials management team. On the system they appear to be in stock, so a reorder is not placed – and this can result in stock-outs and expensive just-in-time orders the next time the item is needed.
Billing
Medical billing systems rely upon full and accurate data being listed on the patient’s chart – if items are missing then they will be missed off the bill.
When this happens there are missing charges on medical billing, which means that the anticipated case funding cannot be secured.
Non documentation costs money.
Now that many healthcare providers are struggling to improve their margins, ensuring full charge capture is such an important task.
Managing UDI Changes in Healthcare Systems
Since UDI changes are common, healthcare providers need to improve their systems and tools in order to cope with everyday challenges such as UDI changes.
Many organizations use POU barcode readers and ERP scanners to record surgical items.
There are all sorts of solutions being put forward to improve surgical data capture, but the two root causes of non-documentation often remain unaddressed.
The two sources of most surgical capture issues are:
- inefficient data capture tools and
- an out-of-date Item Master
Old barcode technology and a reliance on the item master are preventing healthcare organizations from achieving efficient utilization in surgery.
The market just hasn’t been able to provide a quick and easy solution that addresses the root causes of surgical data capture challenges.
Until now.
Finally, new technology is providing a seamless solution for item capture, identification and documentation.
Using technology to solve UDI readability issues
There will always be changes to UDI codes, so healthcare tools need to be designed to deal with this issue.
‘Snap and Go’ is a brand-new way of documenting usage that takes account of the full information on product packaging, not just the barcode.
The managing system processes data collected from the product packaging, and because a much broader range of data has been captured, any barcode change doesn’t prevent item identification and documentation.
‘Snap & Go’ point of use data capture tool makes the charting of utilization a quick and easy task for Circulatory Nurses.

UDI capture using computer vision
‘Snap & Go’ is a brand new POU data-capture tool that uses patented computer vision technology to take a digital image of each product’s packaging.
The image of the product packet is then automatically interpreted and identified using AI cloud technology and a global SKU database.
This takes just 3 seconds per item.
At this point the nurse’s job is done.
Snap & Go ensures quick and accurate data capture, item identification and charting of all items used in surgery.
Snap & Go adds automation to surgical data capture resulting in a quick, accurate and and efficient process.
- Items captured are automatically identified by AI cloud software.
- Full integration with hospital systems – all items captured in surgery are shared with relevant hospital systems, such as the EMR, ERP and MMIS.
- The system is able to identify captured items that don’t have a correct Item Master listing. An exceptions report is generated, containing the updated UDI details, enabling the organization to maintain their Item Master.
The combination of image-recognition, AI and ML technology is changing the way that we capture, identify and record UDI data during surgery.

Takeaways on the benefit of POU data capture using computer vision technology
‘Snap & Go’ uses image recognition and AI technology to achieve accurate charting on the EMR:
- Capturing utilization data is no longer reliant on a correct listing in the Item Master. Quite the opposite, data from ‘snapped items’ helps to update and maintain the Item Master.
- The nurse’s responsibility is now limited to digitally ‘snapping’ each product – item identification and documentation becomes an automated task.
- Reduced time spent on supply chain admin during surgery, freeing up the Circulating Nurse to focus on patient care.
- Accurate surgical documentation boosts hospital or surgery center performance, enhances patient safety, supports better inventory management and optimizes reimbursement.
UDI PI and DI data capture at the point of care
We can see that both the PI and DI section of the UDI code is subject to change and that when a code alters it adds uncertainty and complication to the successful tracking of that item. In particular:
- Changes to the UDI code of medical devices and implants trigger time-consuming workarounds and risk non-documentation.
- Busy nurses are spending far too much time trying to chart all the items used in surgery and sometimes don’t succeed in their task.
- Non documentation is a patient safety risk that makes it hard to track and trace patients affected by product recalls.
- Items that aren’t on the patient’s chart are missed off medical billing, resulting in the underpayment of the case.
- Many healthcare organizations don’t have faith in their POU data capture tools and compensate for this with post case documentation audits. This may result in identifying items missing from the patient chart, but it also adds to the time and cost deficit caused by inadequate tools and a broken system.
The causes of unrecorded UDI data need to be addressed.
It’s time to introduce the next generation of POU data capture tools.
Get ready to discover ‘Snap & Go’.
Contact us to put an end to system errors during surgical charting.
*1 The GTIN Management Handbook:






