What’s inside:
Healthcare providers need to act promptly after receiving recall notifications for medical devices and implants. This article will look at:
- The importance of medical device recall management
- Best practice in managing medical device and implant recalls
- Why automated pre-consumption recall alerts are a gamechanger
- The role of technology in improving patient safety and preventing litigation.
Healthcare organizations need to spring into action when an FDA product recall is received. It’s a race against time to identify all those patients affected by the recall and to ensure that they are given prompt medical advice that could save their life!
What is medical device recall management?
Unfortunately, product defects are a fact of life and when they occur urgent action is required. There are different reasons for product recalls, and these dictate the size and scope of the recall.
- It may be that there is an emerging issue relating to product design, which could result in the full withdrawal of the product.
- Software issues may be affecting implant performance.
- The issue may not be product related at all but may concern mislabeling.
- Products could be having adverse effects on patients.
- Another scenario is that it’s just a faulty batch, affecting specific lot numbers.
FDA Recall Statistics
- Medical device recall events rose from 837 in 2021 to 932 in 2022 – a rise of 11.4%
- In 2022, the FDA issued 70 Class I recalls, compared to an average of 47 over the previous five years.
- In Q1 2023 the number of medical device recalls increased by 4.6 percent to 252 events.
(Data sources at end)
The management of medical device and implant recalls is a patient safety issue that every healthcare organization needs to be prepared for.
Medical negligence – litigation around implant recalls
Manufactures are most likely to be sued for medical device defects, however organizations and physicians are also at risk.
It is therefore important to have systems and procedures in place that, as well as safeguarding patients, protect healthcare providers from litigation.
So, what constitutes negligent practice?
The following scenarios all put the hospital or physician at risk of very expensive litigation:
- Failing to take appropriate action after the issuing of a recall notice, if that failure results in the injury or death of a patient.
- Not keeping accurate records, causing delays to a patient receiving care.
- Surgical use of a recalled implant or medical device is negligent practice.
For these reasons it is crucial to have an effective implant tracking system in place, as well as the tools to achieve complete and accurate point of care documentation. It should be easy to interrogate the data stored.
Medical malpractice in a hospital administration issue
Recall management is an administrative responsibility and failure to have robust processes in place will open up the healthcare organization to litigation.
There have been legal cases against hospitals who have used items which should have been recalled.
In one particular recall litigation case the hospital tried to get the case dismissed on a technicality relating to medical malpractice pre-trial notifications, but the court confirmed that the claim centered on the lack of administrative policies and the failure of staff to respond to a recall notification, and so the case proceeded.
In this particular case, the use of a blood thinner called herapin, which had been recalled months prior to the surgery yet remained in the hospital’s active inventory, sadly resulted in amputations for the affected patient.
This clarification by the courts reinforces the importance of administrative processes to support top quality patient care, organizational compliance and risk reduction.
The importance of accurate recall management
Effective medical implant recall management requires two main tasks:
- PROACTIVE – Preventing consumption: Locating and removing any recalled items that are stored in your supply storage spaces.
- REACTIVE – Tracing and tracking patients: Identifying and contacting all patients who have already consumed the affected item.
Although these tasks appear to be simple, many healthcare providers struggle to gather the full information required for smooth recall management.
It comes down to two types of data – inventory information and patient records.
Let’s look a little closer at these two requirements:
- Item Identification. At the time of a product recall, health systems, hospitals and ambulatory centers may have the affected item in stock. Their task is to find all items and prevent them from being accidentally used in surgery.
- Patient Identification. Providers need to identify the group of patients affected by the recall. This group could be all patients who received the implant, or a subset of the group, such as those that received a specific batch. Once the affected group has been identified, clinical staff are tasked with making prompt contact with each patient, in order to minimize the harm caused by the faulty implant.
In addition to protecting patients, there are also PR and legal reasons for healthcare organizations to get recalls rights.
- Avoiding Litigation: If not handled correctly, medical implant and drug recalls can lead to legal action against healthcare providers. Effective recall management can help mitigate legal risks and protect healthcare providers from liability. Product recalls are a fact of life, but slow, ineffective handling of a recall could be negligence.
- Maintaining Patient Trust: Patients expect healthcare providers to prioritize their safety and well-being. Product recalls cause alarm among the public and need to be handled very carefully. By demonstrating an effective medical implant recall management system, healthcare providers can strengthen patient trust and loyalty.
So, how can healthcare providers improve their handling of product recalls?
Key elements of effective medical implant recall management
Organizations looking at best practice in medical implant recall management need to focus on several key elements, including:
- Monitoring recall announcements: Healthcare providers need to stay up to date with new recalls and safety alerts.
- Quick identification of items: Systems need to be in place to check if there are any recalled items in stock and if so, to locate every item.
- Quick identification of patients: Healthcare providers need to be able ‘track and trace’ every patient who consumed the recalled item.
- Patient follow up: Once the group of patients who consumed the medical device has been identified, the lead physician will need to communicate with each patient, discuss the associated risks and any necessary follow-up care, which may involve the correction or removal of the implant, or could result in no action at all.

How technology is aiding recall management
Technology is transforming the way that healthcare providers are responding to medical implant recalls.
Hospitals and ambulatory care centers are leveraging health-tech solutions to improve their medical device recall management.
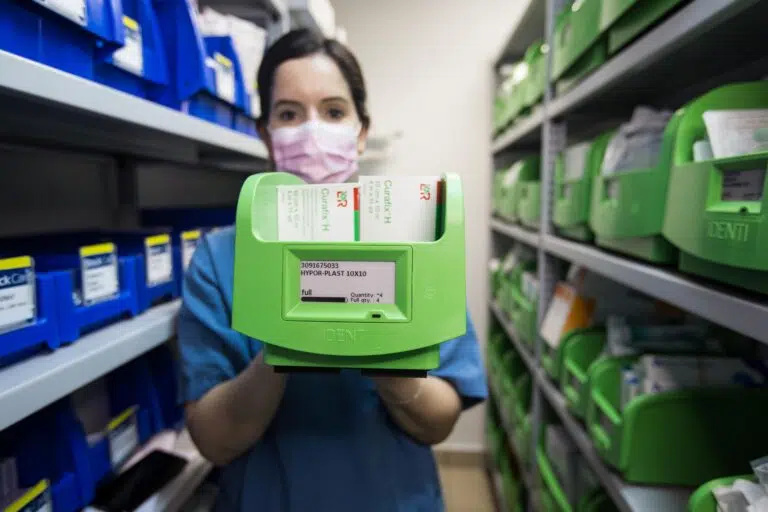
New innovative technology is now available to improve both implant tracking and pre consumption product checking – two crucial foundations for effective product recall management.
There is a stark difference between the manual and automated handling of recalls, as summarized in the chart below.
The information on automated systems will vary depending on their sophistication, the details we use are based on our own products, Total Sense, a smart cabinet suitable for medical devices, implants and other consumables, and Snap & Go, a point of use implant documentation system.
| Element | Description |
| Recall Monitoring | Manual: There needs to be a process that ensures ongoing awareness of new recalls and safety alerts, as well as a way to check whether any of these items are in stock.
Automated: The Total Sense smart cabinet has a managing system that hosts a centrally updated recall list. The system compares this list to the stock at hand, to see if there is a match. Email alerts are generated if there are any recalled items in stock, giving the healthcare provider complete peace of mind. |
| Product Removal | Manual: Manually tracking down every recalled item is a time consuming and error prone process. If there is a specific batch that is being recalled, then each affected product will need to be carefully checked. There’s no room for error, if any items are left in stock, they could be accidentally used – a huge patient risk.
Automated: The Total Sense provides real-time inventory vision and can identify exactly where the affected products are located. Staff can be signposted to the exact location of every recalled stock item, so that these can be swiftly removed from the shelves. |
| Patient Identification | Manual: It’s a challenge to identify all affected patients using manual medical records, implant books or outdated systems. Ultimately, manually handling recalls is a long, inaccurate process that risks patients remaining unidentified.
Automated: Full, accurate, digital documentation at the point of care makes it quick and simple to identify patients who consumed the recalled medical device. |
| Outcome: | Manual: Manual systems are slow and inefficient making it hard to identify recalled stock items and patients who consumed specific products. Manual recall handling poses a high risk to patient safety and litigation.
Automated: Precise identification of both recalled stock items and affected patients – for robust and speedy recall management. |
Medical device recall best practice
Effective medical implant recall management is essential for patient safety and well-being. Healthcare providers should have systems in place that accurately track their medical devices and implants – both prior to usage and after consumption.
Automated implant tracking systems and point of use documentation systems ensure that best practice recall management is in place.
RFID smart cabinets are commonly used to track implants and the best cabinets are able to support easy recall management.
The point of care is probably the most challenging data-capture point in any healthcare organization but it’s also the most crucial.
The surgical setting is the last opportunity the organization has to check items before consumption.
Tools that provide recall alerts at the point of care provide a critical layer of protection.
Put simply, if utilization is not being accurately recorded, then the patient is not being properly protected.
Healthcare providers looking to improve recall management can use technology as a safety net. Automated implant management solutions are a quick and precise tool that lightens the weight of responsibility felt by nurses, physicians and management.
Automating recall management using high quality inventory management solutions ensures that healthcare providers can minimize harm to patients, avoid litigation, and maintain patient trust.
Contact us to find out how technology can help you to improve your recall management.
references:
FDA Data: