What’s inside:
The Hospices Cibils de Lyon, is part of the Groupement Hospitalier Sud (GHS) and is made up of 14 public, multi-disciplinary hospitals. This case study looks at the introduction of an automated implant and medical device tracking system, and covers:
- The background and challenges faced by the Hospices Civils de Lyon.
- The goals of the implant management project
- The solution identified for enhanced implant / medical device tracking and control.
- Project implementation
- Results and outcomes, including data.
Background
The Hospices Civils de Lyon (HCL) was seeking enhanced automation and precision for the management and traceability of implanted medical devices. Medical device tracking is a major issue in the French healthcare sector.
In modern healthcare facilities, effective management and traceability of implants and medical devices are paramount for ensuring patient safety, regulatory compliance, and operational efficiency. In the European healthcare market, as well as in the rest of the Western world, healthcare providers face challenges in maintaining accurate records of what are called durable medical supplies (DMS) and durable medical instruments (DMI) due to the complexity of inventory management processes and regulatory requirements.
In the European healthcare services market, the hospital pharmacist plays a crucial role in the supply chain. They are responsible for procuring health products, storing them in the pharmacy unit of use (PUI), and dispensing them to care units and operating rooms. The hospital’s pharmacy is required to maintain regulatory standards that mandate robust traceability of DMIs, requiring healthcare establishments to maintain detailed records of product batches, serial numbers, and expiration dates to facilitate product recalls and ensure patient safety.
Regulatory requirements mandate robust traceability measures for medical devices and implantable inventory, which are known in Europe by their official name durable medical instruments (DMI) or durable medical equipment DMS, to ensure that healthcare facilities can track these devices throughout their lifecycle. This includes the ability to trace DMIs implemented within the establishment and access health traceability data for IMDs installed in individual patients.
The European Regulation 2017/745 on Medical Devices introduces mandatory measures to strengthen traceability and post-marketing surveillance, including the implementation of Unique Device Identifiers (UDIs). UDIs are alphanumeric codes affixed to devices at various levels of packaging and registered in the Eudamed database, facilitating traceability across the distribution chain. This EU directive is similar to FDA UDI regulations, established in 2013, which includes the UDI, device labeling for human and machine reading, database submission to the FDA’s GUDID, and compliance dates for implementation.
Eudamed serves as a central database for medical devices, enabling coordination between regulatory authorities and the sharing of critical information. Additionally, the regulation mandates the provision of implant cards by manufacturers, ensuring comprehensive traceability of DMIs from production to end-user, in compliance with regulatory standards.
EU implant and medical device tracking regulations
The European Regulation 2017/745 on Medical Devices, commonly known as the EU Medical Device Regulation (MDR), is a regulatory framework established by the European Union to govern the marketing and distribution of medical devices within its member states. This regulation aims to ensure the safety, performance, and quality of medical devices while enhancing transparency and traceability throughout the supply chain.
The EU MDR introduces stricter requirements for the approval, certification, and surveillance of medical devices compared to previous regulations. It includes provisions for the classification of devices, conformity assessment procedures, post-market surveillance, and vigilance reporting.
One of the key components of the EU MDR is the implementation of a Unique Device Identification (UDI) system. This system assigns a unique identifier to each medical device, allowing for better traceability and easier recall of products in case of safety issues or defects. The UDI system is designed to enhance transparency, streamline regulatory processes, and improve patient safety.
Overall, the European Regulation 2017/745 on Medical Devices, or the EU MDR, represents a comprehensive regulatory framework aimed at ensuring the safety and effectiveness of medical devices in the European market, while also harmonizing regulations across member states to facilitate trade and innovation.
Goals
The Northern Hospital Group Civil Hospices of Lyon sought a solution for managing its implantable inventory in the Interventional Cardiology Department. This specialized unit is dedicated to diagnosing and treating cardiovascular conditions using minimally invasive procedures, led by experienced cardiologists and supported by skilled healthcare professionals. Offering services like angioplasty, stenting, and cardiac catheterization, patients receive personalized care and access to cutting-edge technology for accurate diagnosis and effective treatment. Committed to high-quality cardiovascular care, the department focuses on improving patient outcomes and enhancing quality of life.
Implementing RFID technology for managing implanted medical devices aims to enhance efficiency, security, and traceability in healthcare services and operating room theaters. Through RFID technology and professional software solutions, the hospital aims to achieve several key goals:
- Enhancing security and traceability throughout the implant lifecycle
- Complying seamlessly with the strict requirements of the EU Medical Device Regulation by recording batch numbers and serial numbers for a digitalized database
- Optimizing inventory management processes to eliminate waste and inefficiency in demand planning
- Reducing pharmacy paramedical time dedicated to supply tasks
- Implementing cost-effective and safe expiry management of implantable devices
- Ensuring interoperability with existing software systems such as the GEF software, WMS, and health traceability systems.
By achieving these objectives, healthcare facilities can enhance patient safety, streamline operations, and comply with regulatory requirements.
“Before the deployment implantable medical devices were entirely managed by the ide team, very often on-call, at night, ‘when we had time’, without systematic stock checking. Today it has transformed into a digital system where we have full control and can proactively anticipate and respond to product expirations.”
Agnes Henry, Hospital Pharmacist, LYON University Hospital
Solution
With assistance from Promedo, a local channel partner specializing in the French health market and marketing IDENTI’s products, a thorough diagnostic process was conducted. The chosen solution to the challenges of managing implanted medical devices involves deploying TotalSense RFID cabinets, a mobile app and handheld RFID scanners, integrated with the IDENTIPlatform, cloud-based software powered by AI, for comprehensive traceability and inventory management.

The RFID cabinets are equipped with RFID tags that store unique device identifiers (UDIs), enabling healthcare providers to track the movement and usage of IMDs in real-time. The mobile RFID systems consist of handheld RFID scanners, and a mobile app that streamline the inventory, and replenishment processes for all items located on storeroom shelves. By scanning RFID tags, healthcare providers can record product information, monitor expiration dates, and automate inventory replenishment tasks, thereby improving inventory accuracy, reducing stockouts, and enhancing operational efficiency.
Implementation
The implementation process of RFID technology involves several steps, including tagging products upon receipt at the pharmacy, delivering them to designated service areas, storing them at the point of care and then using them as needed. Regular, automated stock inventories are conducted, followed by ordering and replenishment as triggered by the automated system alerts.
The new workflow streamlines inventory management processes, enhances collaboration between pharmacy and service departments, and ensures seamless integration with existing workflows and software systemsThe process begins with reading the GTIN code (barcode) of the product upon receipt and pairing it with a tag via the tagging station connected to the IDENTIPlatform Software. This tag includes the batch, serial number, and expiration date of the product. Inventory is monitored by scanning tags in the Coro (Coronarography) storage room using the mobile reader. The TotalSense application detects new DMs received and identifies all those that have been removed by comparing data from the new ‘stock take’ to previous inventory data. The IDENTIPlatform software extracts a list of DMIs reaching their expiry date and also reports on all items with a current stock level that has fallen below the preset minimum stock threshold.
“Concerning DMS, TotalSense mobile RFID systems are preferred to other solutions according to the criteria of inventory optimization, and compliance with safety regulations. However, one of the major advantages of the RFID solution is the traceability of all DMI movements without the need for human intervention.”
Marie Levêque – French public hospital purchasing advisory board, UNIHA

Results and Outcomes
The successful implementation of Total Sense RFID Solution by Northern Hospital Group Civil Hospices of Lyon in Interventional Cardiology has revolutionized inventory management and traceability of implanted medical devices. By utilizing RFID technology and professional software solutions, the hospital was able to enhance efficiency, security, and compliance while maintaining the highest standards of patient care.
The deployment of RFID technology for managing implanted medical devices has yielded significant results in terms of inventory optimization, cost savings, and operational efficiency.
UDI EU Medical Device Regulation:
The documentation compliance rate has surged to 91%, facilitated by the collection of all GTINs and the creation of a digital catalog featuring batch history and serial numbers in the database. This enables swift recall retrieval using IDENTIPlatform‘s cloud capability, ensuring compliance with the UDI EU Medical Device Regulation by recording batch numbers and serial numbers for a digitalized database.
Stock management and economic impact:
Before the deployment of the TotalSense system, the management process was described by Agnes Henry, Hospital Pharmacist, as being “entirely managed by the clinical team, very often on-call, at night, ‘when we had time’, without systematic stock checking.” In 2019, for the coronary angiography service, the annual expenditure on durable medical supplies (DMS) amounted to €2,067,826, representing 11.6% of the hospital’s total expenditure on medical supplies. At that time, 49% of the SKUs were overstocked, exceeding twice the monthly consumption. Following the implementation of the TotalSense system, the hospital experienced significant improvements. There was a notable 37% reduction in stock quantity. Additionally, at the time of evaluation – 8 months post introduction of the system, the pharmacy team indicated a decrease of over 50% in the number of overstock SKUs which today is even lower, as the inventory has dramatically reduced and is now balanced.
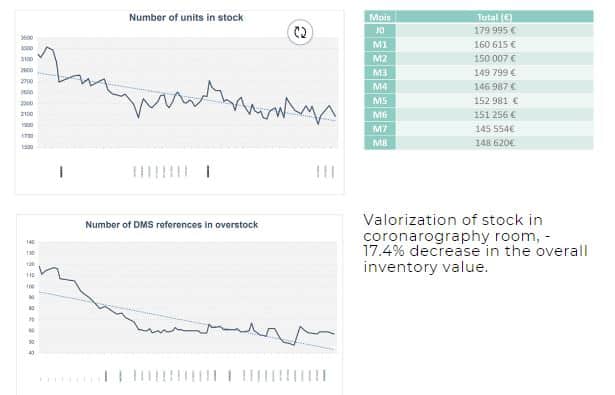
Expiry management:
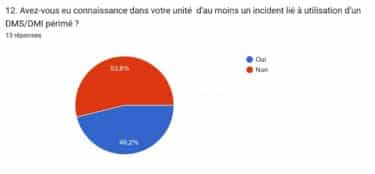
Prior to the system deployment, 52% of pharmacists were aware of incidents involving expired durable medical supplies (DMS) or durable medical instruments (DMI), highlighting the challenge of managing inventory. However, since the implementation of the system, there have been no reported incidents of expired DMS/DMI. Additionally, before system deployment, nearly half of the Cardio services experienced incidents related to the use of expired medical devices (MDs). With the introduction of RFID technology, there has been better anticipation and management of expiration dates. The IDENTIPlatform software facilitated full medical device tracking, enabling the extraction of a list of MDs approaching their expiry date and shows when a DMS or DMI quantity has fallen below the minimum stock threshold.
Workflow efficiency:
The system has strengthened the link between the Interventional Cardiology Department and the Pharmacy Departments, optimized storage areas, reduced inventory time, and improved proactive expiry management. The Cardio Service team now spends less time on supply chain tasks, with a reduction in time spent on orders by 3 hours per week, allowing for a refocusing on care activities. These results demonstrate the effectiveness of RFID technology in enhancing inventory management and traceability in healthcare settings, leading to improved patient safety and operational outcomes.

Conclusion:
The Hôpital de la Croix-Rousse introduced TotalSense RFID Smart Cabinet and Mobile Implant and medical device tracking technology. After analyzing inventory data they identified many clinical, operational and financial benefits. .
The fully automated implant management system provided the item level data they needed to gain full visibility and control of their durable medical supplies and instruments. This ensured they were able to digitally track this inventory and demonstrate full compliance with European medical device and implant regulations.
The Pharmacy Department is now able to closely monitor all inventory after it is dispatched to the various departments, retaining full oversight and control.
If you organization is seeking enhanced inventory visiblity and item level tracking, find out more about TotalSense Implant Management Solution.






